Our pipeline
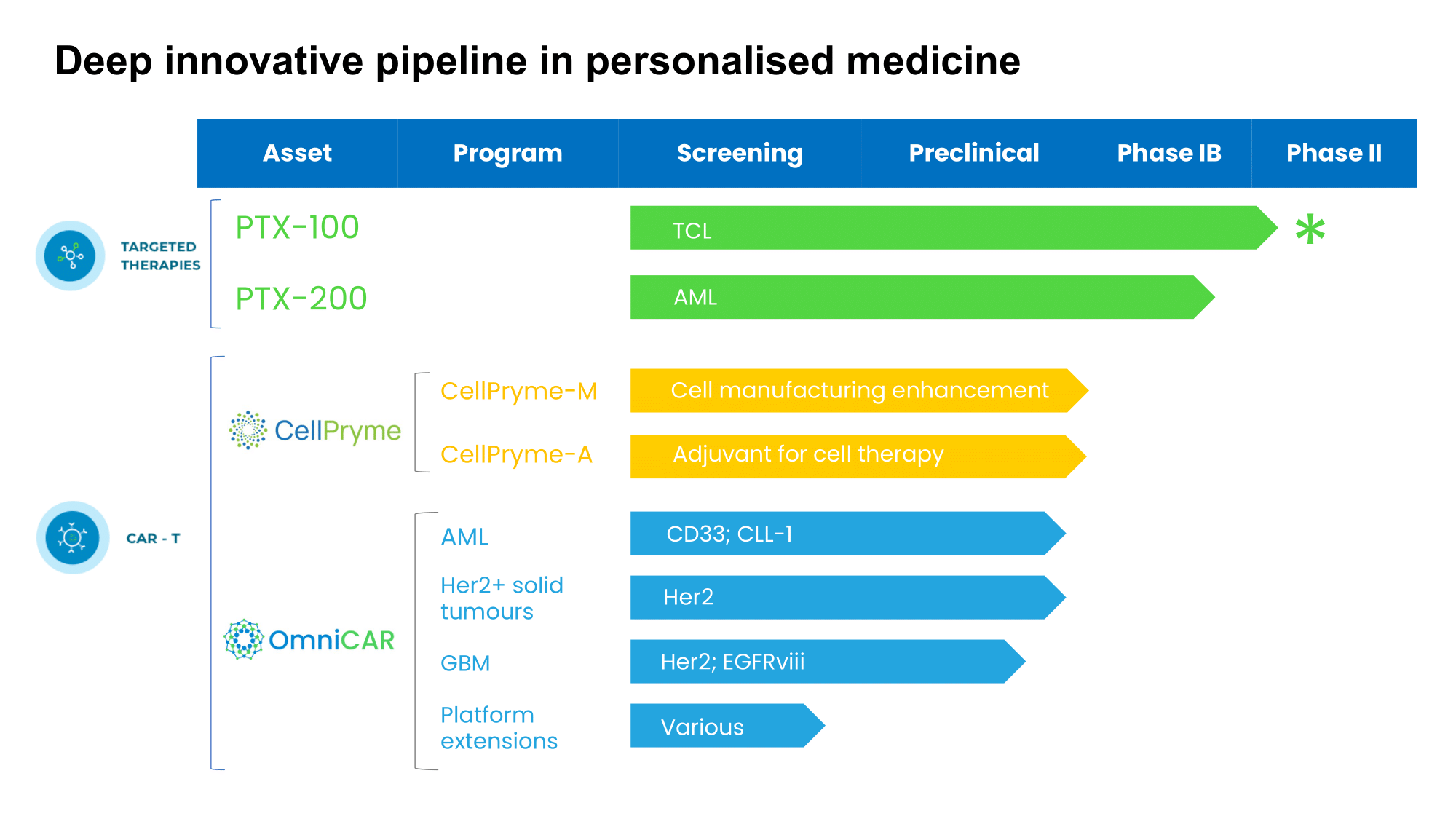
Prescient has a broad pipeline of later stage and emerging assets in next generation targeted and cellular therapies and spanning a range of different cancers.
Prescient has licensed technologies from and collaborates with world-leading cancer centres in the US and Australia.
Our technologies
Cell therapy: OmniCAR
The OmniCAR platform seeks to overcome the challenges of conventional CAR-T therapy including manufacturing, safety and reliability. OmniCAR is a modular, universal CAR platform based on technology licensed from UPenn and Oxford University. It allows unprecedented control and flexibility over current generation CAR-T approaches
Cell therapy: CellPryme
CellPryme is a high-performance cell therapy enhancement platform that can improve CAR-T efficacy. The CellPryme platform consists of two distinct components which can be used separately but have significant synergies when used together. CellPryme-M can be briefly added to standard manufacturing processes. CellPryme-A is used as an adjuvant alongside cellular immunotherapy.
Targeted therapy: PTX-100
PTX-100 is a first in class compound with the ability to block an important cancer growth enzyme, thereby disrupting the oncogenic Ras pathway. PTX-100 is now in a Phase 1b expansion cohort study in T cell lymphomas, where it is showing encouraging efficacy and safety. The US FDA has granted PTX-100 Orphan Drug Designation for all T cell lymphomas.
Targeted therapy: PTX-200
PTX-200 is a novel PH domain inhibitor that inhibits an important tumor survival pathway known as Akt, which plays a key role in the development of many cancers, including leukemia; breast cancer and ovarian cancer.
