Prescient Therapeutics is developing personalised medicine approaches to cancer, including cell therapies and targeted therapies.
Targeted Therapy
PTX-100
PTX-100 is a first in class compound with the ability to block an important cancer growth enzyme, thereby disrupting the oncogenic Ras pathway. PTX-100 is now in a Phase 2a clinical study in refractory/relapsed Cutaneous T Cell Lymphoma. The US FDA has granted PTX-100 Orphan Drug Designation for all T cell lymphomas, Investigational New Drug (IND) status and Fast Track Designation.
Cell Therapy
CellPryme
CellPryme is a high-performance cell therapy enhancement platform that can improve CAR-T efficacy. The CellPryme platform consists of two distinct components which can be used separately but have significant synergies when used together.
CellPryme-M
Prescient’s novel, ready-for-the-clinic, CellPryme-M technology enhances adoptive cell therapy performance by shifting T and NK cells towards a central memory phenotype, improving persistence, and increasing the ability to find and penetrate tumours. CellPryme-M is a 24-hour, nondisruptive process during cell manufacturing. Cell therapies that could benefit from additional productivity in manufacturing or increased potency and durability in-vivo, would be good candidates for CellPryme- M.
CellPryme-M benefits
The cells produced from the CellPryme-M process have many favourable characteristics required from more effective cell therapies, including:
- 50% more central memory T cells, a highly clinically relevant sub-type;
- Double proportion of CD4+ helper T cells, for synergy with effector T cells;
- Significantly more chemokine receptors, important for tumour trafficking and tumour penetrance, especially important in solid tumours; and
- Greater genomic stability and DNA repair for enhanced self-renewal.
Importantly, the superior cell phenotype of CellPryme-M cells does not come at the expense of effectiveness, with T cells retaining their potency with no increased safety risks due to higher cytokine release
CellPryme-A
CellPryme-A is an adjuvant therapy designed to be administered to patients alongside cellular immunotherapy to help them overcome a suppressive tumour microenvironment. CellPryme-A significantly decreases suppressive regulatory T cells; increases expansion of CAR- T cells in vivo; increases tumour penetration of CAR-T cells. CellPryme-A improves tumour killing and host survival of CAR-T cell therapies, and these benefits are even greater when used in conjunction with CellPryme-M pre-treated CAR-T cells.
CellPryme-A benefits
- Enhances tumour killing by conventional CAR-T cells; especially strong benefits when used in conjunction with CellPryme-M
- Improved host survival
- Reduces problematic Treg cells by 66%
- Increases ability of T cells to penetrate solid tumours
- Cytotoxic T cells by 400%
- Helper T cells by 300%
- Dramatically increases ability of CAR-T cells to expand within the host
- Doubles CAR-T cell expansion
- When used in conjunction with CellPryme-M:
- Cytotoxic T cells increased by 900%
- Helper T cells increased by 600%
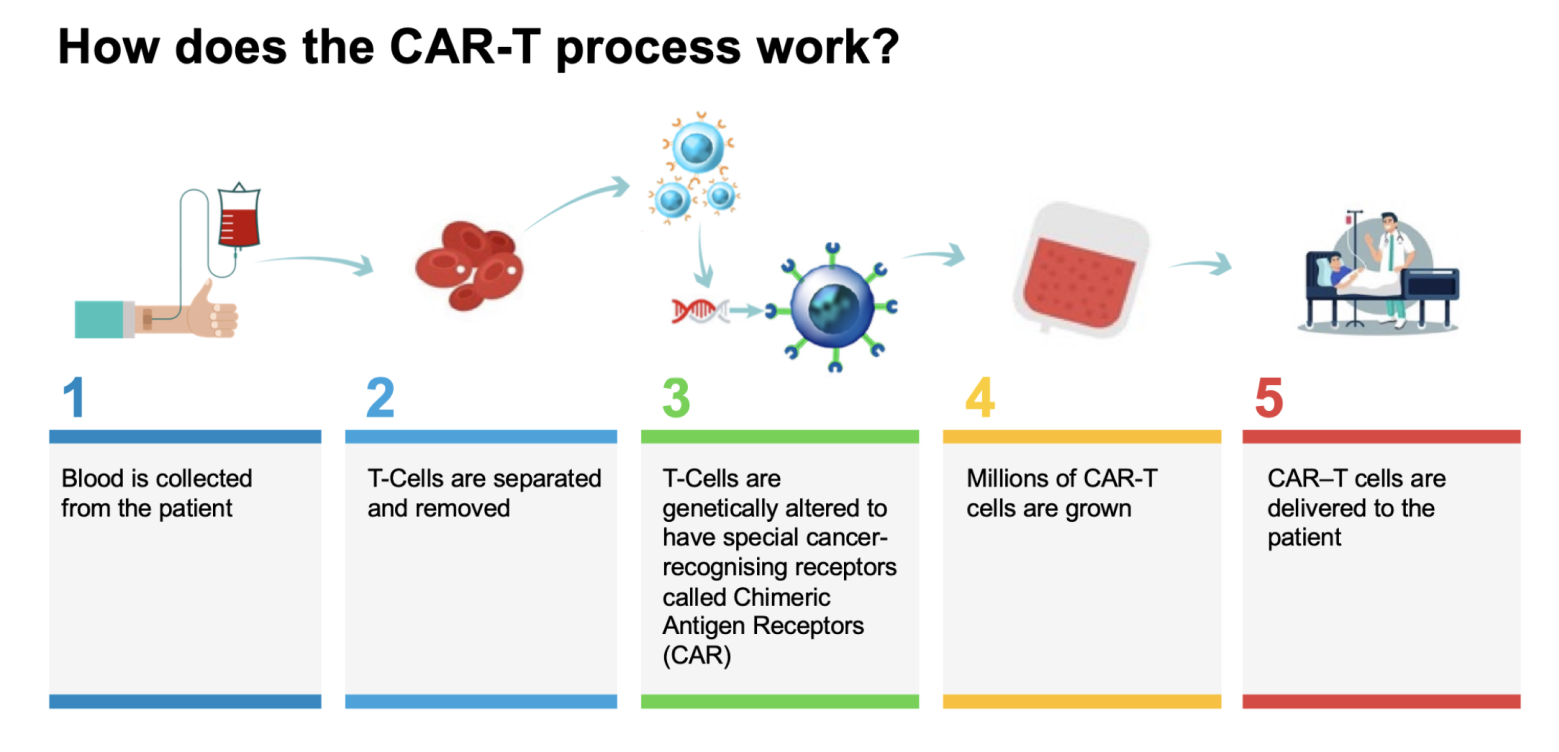
What is CAR-T therapy?
CAR-T – Chimeric Antigen Response T-Cell therapy – is a new type of therapy that has shown stunning responses in certain blood cancers and may be replicable in other types of cancers as well. It is ‘the most personalised medicine’.
CAR-T therapy works by taking immune cells from the patient’s body that fight infection – called T-cells – and training them outside the body to recognise cancer cells. They’re then grown in large numbers and reinjected into the patient whereby they can track down, fight and kill the patient’s cancer cells. CAR-T cell therapy has been extensively tested on certain blood cancers, producing some truly incredible results.
However, CAR-T therapies currently have a number of drawbacks that must be overcome to bring this revolutionary class of medicine to more cancer patients. These challenges include cost, safety, a lack of post infusion control, longevity and limited ability to target multiple cancer antigens. Prescient has two platform technologies to overcome these challenges: OmniCAR and CellPryme.
OmniCAR
OmniCAR is a next-generation, universal CAR platform that is controllable, with “plug and play” capabilities to enable a wider range of cancers to be targeted. OmniCAR: enables controllable T-cell activity and multiantigen targeting with a single cell product. OmniCAR’s modular CAR system decouples antigen recognition from the T-cell signalling domain. It is the first universal immune receptor allowing post translational covalent loading of binders to T-cells. OmniCAR is based on technology licensed from Penn; the SpyTag/SpyCatcherbinding system licensed from Oxford University; and other assets.
The targeting ligand can be administered separately to CAR-T cells, creating on-demand T-cell activity post infusion and enables the CAR-T to be directed to an array of different tumour antigens. OmniCAR provides a method for single-vector, single cell product targeting of multiple antigens simultaneous or sequentially, whilst allowing continual re-arming to generate, regulate and diversify a sustained T-cell response over time.
Video: OmniCAR explained
OmniCAR benefits
OmniCAR offers control and flexibility that is beyond the reach of current generation CAR-T cell therapy. OmniCAR is expected to provide clinicians with unprecedented control over safety aspects of CAR-T, by allowing them to tune CAR-T cell activity either up or down post-infusion, and also enables them to switch off CAR-T activity altogether by ceasing administration of the targeting ligand (i.e. a built-in “kill switch”).Unlike conventional CAR-T, which is limited to a single target, OmniCAR-T cells are able to be armed against multiple targets sequentially or simultaneously by simply switching out the targeting ligand, which will be especially important in CAR-T making headway into the large field of solid tumours.
As the industry attempts to drive down the cost and time of delivering CAR-T therapy to patients, OmniCAR’s flexibility and control will be an increasingly valuable tool, by removing the need for multiple manufacturing runs per patient, and its compatibility with allogeneic (“off the shelf”) T-cells. OmniCAR is potentially compatible with many other CAR approaches including NK cells; macrophages and stem cells.
OmniCAR programs
Prescient is developing OmniCAR programs for next-generation CAR-T therapies for Acute Myeloid Leukemia (AML); Her2+ solid tumours, including breast, ovarian and gastric cancers; and glioblastoma multiforme (GBM).

